
VETERINARSKI ARHIV 68 (2), 39-49, 1998
ISSN 0372-5480
Printed in Croatia
Ultrastructural and toxicological investigations in spontaneous cases of porcine nephropathy in Bulgaria
Stoytcho D. Stoev1*, Nely Grozeva1, and Benedicte Hald2
1Department of Pathomorphology, Faculty of Veterinary Medicine,
Thracian
University, Stara Zagora, Bulgaria
2Department of Veterinary Microbiology, Royal Veterinary and Agricultural
University,
Frederiksberg, Denmark
* Contact address:
Prof. Dr. Stoytcho Stoev,
Department of Pathomorphology, Faculty of Veterinary
Medicine, Trachian University, Students campus, 6000 Stara Zagora, Bulgaria,
Fax: 359 42 74067/39546
STOEV, S. D., N. GROZEVA, B. HALD: Ultrastructural and toxicological investigations in spontaneous cases of porcine nephropathy in Bulgaria. Vet. arhiv 68, 39-49, 1998.
ABSTRACT
The kidneys of 50 normally slaughtered pigs exhibiting lesions of the type "enlarged mottled or pale kidneys" during meat inspections were investigated ultrastructurally with the aim of elucidating the nature of nephropathy in Bulgaria. The typical early ultrastructural changes were observed in the epithelium of proximal tubules: as well as characteristic mitochondrial lesions such as reduced cristae and lost membrane integrity, myelin-like figures and electron dense formations in many mitochondria, specific intranuclear inclusions, as well as degenerative changes in capillary endothelium, were established. The later stages were characteristic, with a large number of collagen fibrils in the interstitium and thickened basement tubular membranes. Nephrotoxic mycotoxin ochratoxin A was found in 100% of investigated serum samples collected from the same pigs.
Key words: nephropathy, ochratoxin A, ultrastructural changes, pig, kidney, Bulgaria
Introduction
A nephropathy in pigs with characteristic macroscopic changes of the type "mottled or pale enlarged kidneys" has been frequently identified at meat inspection in Bulgaria. During the last years such nephropathy was very often observed in the spring at meat inspection of slaughtered animals (STOEV, 1992; STOEV et al., 1994). Ultrastructural and toxicological investigations of kidneys and blood of normally slaughtered pigs were carried out with the aim of elucidating the nature of the observed nephropathy.
Materials and methods
The investigations were carried out during the spring-summer of 1994 and included kidney samples from 50 normally slaughtered pigs exhibiting characteristic colour and size renal changes, such as "mottled or pale enlarged kidneys", during meat inspection. The examined kidneys represented about 20% of the damaged kidneys ("pale or mottled") from 5 batches of pigs from five large-scale pig farms with known porcine nephropathy in north-east region of the country, along the Danube basin.
Kidney tissues were fixed in 2% osmium tetroxide for 1 hour and embedded in "Durcupan". The sections were stained with uranyl acetate and lead citrate and examined under Opton 10C and Jeol 100C electron microscopes.
Five serum samples were collected from each affected farm studied (a total 25 samples from 5 farms) during the spring of 1994. The samples were taken at slaughter time during meat inspection and came from pig batches (from the same affected farms) exhibiting macroscopical lesions in kidneys of the type "enlarged mottled or pale kidneys" in a high percentage (about 30-50%) of slaughtered pigs. The samples were taken from pigs exhibiting the described macroscopical lesions and were frozen at 20 °C until toxicological examination. The samples were analysed with ochratoxin A by the HPLC (high performance liquid chromatography) technique.
Results
The frequency of observed nephropathies varied significantly in the examined farms. From 30-40, to 80-90% of slaughtered pigs from these farm had kidney lesions of the type "enlarged mottled or pale kidneys" established during meat inspection. Their duration also differed: from 2-3 up to 7-8 months. As a rule, nephropathies were observed predominantly during the spring-summer period in wet years.
Ultrastructural investigations revealed that the major lesions were to be observed in the epithelial cells of the proximal tubules. The many cell organelles in the damaged epithelial cells exhibited lost membrane integrity, and the brush border was reduced in height and density. The quantity of cell organelles (mitochondria) in the part of the damaged epithelial cells was decreased (Fig. 1). In some cases cellular components were condensed to an extent that excluded identification of subcellular elements. Aggregation of cellular debris was observed in the lumen of some dilated and damaged proximal tubules. Large apical vesicles were often seen in some preserved epithelial cells (Fig. 2).

Fig. 1. Electron micrograph of the basal part of the proximal tubular epithelial
cells of a kidney. The quantity of mitochondria and other cell organelles
is reduced in number in the basal part of the damaged proximal tubular
epithelial cells.
× 5,000; scale bar = 2 µm.

Fig. 2. Electron micrograph of the epithelial cells of the convoluted tubules
of a kidney. The brush border is reduced in height and density, or is absent
altogether. Large apical vesicles are often seen in some preserved epithelial
cells.
× 8,000; scale bar = 1.25 µm.
A great number of mitochondria were swollen, with extremely electron-dense formations (Fig. 3), reduced invisible cristae and, in some cases, exhibited lost membrane integrity (Fig. 4). In some cases, various myelin-like figures or a lipid mass was established in the cytoplasm or in some mitochondria (Fig. 5).

Fig. 3. Electron micrograph of the basal part of the proximal tubular epithelial cell a kidney. Many mitochondria are swollen, with extremely electron-dense formations, damaged cristae and lost membrane integrity. × 50,000; scale bar = 0.2 µm.

Fig. 4. Electron micrograph of the apical part of the proximal tubular epithelial cells of a kidney. A large number of mitochondria are swollen, with reduced invisible cristae and, in some cases, exhibit lost membrane integrity. × 20,000; scale bar = 0.5 µm.

Fig. 5. Electron micrograph of the basal part of the proximal tubular epithelial
cell of a kidney. It is seen as a myelin-like figure and a lipid drop in
the cytoplasm.
× 16,000; scale bar = 0.625 µm.
Many nuclei were displayed condensation of chromatin and invagination of the nuclear envelope (Fig 6). In some cases the nuclei were enlarged with decreased condensed chromatin. Rarely, extremely electron-dense formations, surrounded with lesser-electron dense formations, were observed in the nuclei (Fig. 7).

Fig. 6. Electron micrograph of the epithelium of the proximal tubules of
a kidney. It is observed as a nuclei with condensation of chromatin and
invagination of the nuclear envelope. Many mitochondria are swollen, with
damaged cristae.
× 10,000; scale bar = 1 µm.

Fig. 7. Electron micrograph of the middle part of the epithelium of the proximal tubules of a kidney. An extremely electron-dense formation surrounded with a small electron-dense formation in the nucleus. × 10,000; scale bar = 1 µm.
The number of peroxisomes with a fine granular material, as well as one or two paracrystalline plates in the membranes, increased in many epithelial cells (Fig. 8). Phagolysosomes with extremely electron-dense formations were frequent found in the damaged cells of proximal tubules (Fig. 9).

Fig. 8. Electron micrograph of the middle part of the proximal tubular
epithelial cell of a kidney. An increased number of peroxisomes, with one
or two paracrystalline plates in the membranes. Mitochondria with slightly
damaged cristae.
× 25,000; scale bar = 0.4 µm.
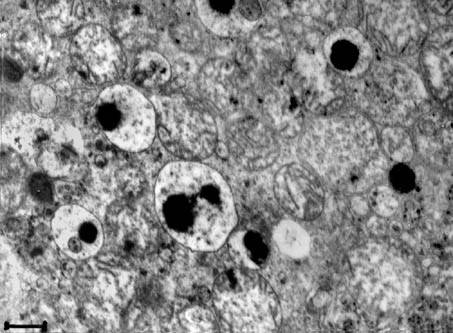
Fig. 9. Electron micrograph of the middle part of the proximal tubular epithelial cell of a kidney. Lysosomes with an extremely electron-dense formation are frequent in the damaged epithelial cells. × 20,000; scale bar = 0.5 µm.
In the later stages a large number of collagen fibrils in the interstitium and thickened basement tubular membranes were observed (Fig. 10).

Fig. 10. Electron micrograph of the basal part of the proximal tubules of a kidney. Thickened basement tubular membranes and a large number of collagen fibrils in the interstitium. × 12,600; scale bar = 0.794 µm.
Similar but slightly pronounced changes were observed in some of the loops of Henle, distal and collecting tubules.
Some fibroblasts in the interstitium showed a slight dilatation of the rough endoplasmic reticulum. A slight oedema was established in the interstitium in some later stages of the disease.
Degenerative changes in the capillary endothelium were frequently observed.
The toxicological investigations of serum samples of pigs from various farms showed that the established average concentration of all investigated serum samples was 60.9±9.19 ng ochratoxin A/ml, whereas the percentage of positive samples was 100% (Table 1).
Table 1. Mean concentration (±SE) of ochratoxin A (OTA) in various pig
serum samples originating from different farms with porcine nephropathy
during the spring of 1994 in Bulgaria
|
Origin of samples |
Number of examined samples |
Positive samples (%) |
Mean concentration levels of OTA (ng/ml) |
|
Sitovo |
5 |
100 |
48.34±6.7 |
|
Dulovo |
5 |
100 |
75.78±19.58 |
|
Nojarevo |
5 |
100 |
53.44±8.32 |
|
Nova Cerna |
5 |
100 |
84.20±41.17 |
|
Cernogor |
5 |
100 |
42.64±3.60 |
|
Mean |
25 |
100 |
60.88±9.19 |
Discussion
Analysis of various ultrastructural changes shows striking similarities between the described porcine nephropathy in pigs and Balkan endemic nephropathy (BEN) in human disease with an as yet unknown aetiology (DIMITROV, 1984; FERLUGA et al., 1991, 1996; HVALA et al., 1993; COSYNS et al., 1994; BOZIC et al., 1995). Major lesions in both diseases are observed in the epithelial cells of proximal tubules and their brush border is reduced in height and density. Mitochondria are reduced in number, are sometimes slightly swollen, with extremely electron-dense formations, lipid droplets, myelin-like figures (predominantly observed in porcine nephropathy in Bulgaria, although unlike that described in Denmark) and reduced invisible cristae. Cell organelles exhibit lost membrane integrity; a large number of apical vesicles and phagolysosomes are often seen; peroxisomes with granular material and one or two paracrystalline plates in the membrane appeared in the cells; many nuclei are with condensation of chromatin and disappearance of the nuclear envelope. At a later stage, a thickening of the basement tubular membranes of the proximal tubules and a large number of collagen fibres in the interstitium dominate the ultrastructural picture in both diseases. The described ultrastructural abnormalities include the occasional findings of lipid droplets, suggesting a degenerative process of a nephrotoxic character.
The lost membrane integrity of the cell organelles may be due to a specific inhibition of the protein synthesis by ochratoxin A, and subsequent impairment of lipoprotein structures in the cells (CREPPY et al., 1983). The lost membrane integrity of the cell organelles presuppose enhanced levels of catalase and various lysosomal enzymes in soluble cell fractions, which could be associated with continuous desquamation and degenerative changes in the epithelial cells of proximal tubules.
On the other hand, ochratoxin A inhibits mitochondrial oxidative phosphorylation by acting as a competitive inhibitor of carrier proteins located in the inner mitochondrial membrane (MEISNER and CHAN, 1974; ELLING et al., 1985). Thus, damaged energy production may determine those early changes in the mitochondria observed in our investigations.
The inhibition of protein synthesis and the damaged energy production in the mitochondria could be considered as the most important factors for degenerative changes in the epithelial cells of proximal tubules where ochratoxin A was detected. Various investigations have suggested that ochratoxin A could enter the plasma membrane in the middle and terminal portions of the proximal tubules through the anion transport pathway (STORMER, 1992) where it realises the observed damage by inhibition of protein synthesis and impairment of energy production in the mitochondria.
The great number of large apical vesicles in some preserved epithelial cells may be a compensatory mechanism for reasorption of some useful substances.
However, at this moment in time we are not sure that the electron-dense formations in the nuclei, surrounded with a small-electron dense mass, as well as myelin figures in the cytoplasm and mitochondria and vascular lesions, are provoked by ochratoxin A. These changes have not been observed in mycotoxic porcine nephropathy (ochratoxicosis) most notably studied in Denmark by ELLING et al. (1985). The described differences in our occurrences of porcine nephropathies are probably due to the some interference between ochratoxin A and other mycotoxins. Accordingly, it is necessary to carry out more investigations and experimental studies for establishing the etiological nature of nephropathy found in Bulgaria.
The toxicological investigations of serum samples of pigs from various farms showed that there was no pronounced correlation between the macroscopical appearance of kidneys and the concentration of ochratoxin A in corresponding blood samples. The established average concentration of all investigated serum samples (60.9±9.19 ng/ml) and the percentage of contaminated samples (100%) showed that the contamination levels of ochratoxin A in the swine serum samples were very high compared to those from other countries (HULT et al., 1980; GOLINSKI et al., 1984).
It has been established that there is a statistical association between concentration of ochratoxin A in various tissues. If necessary, it is possible to calculate the residue levels of ochratoxin A in kidney, liver, muscle, fat on the basis of the ochratoxin A content in blood by regression analysis (MORTENSEN et al., 1983). Calculated by this equation the average concentration of ochratoxin A in kidneys (4µg/kg) did not exceed the maximum tolerance for ochratoxin A in kidneys of pigs slaughtered for meat established in Denmark (10 µg/kg). On the other hand, we have to draw attention to circumstance where a large part of the toxin is eliminated from the tissues when pigs are kept on a toxin-free diet during several days before slaughter. Also, it is very likely that the possible involvement of other nephrotoxic mycotoxins would be realised.
Conclusion
Independent of the circumstances under which the mean etiological agent of the observed mycotoxic nephropathy in Bulgaria is supposed to be ochratoxin A, it is very likely that we would realise some synergistic effects between ochratoxin A and various other nephrotoxic mycotoxins produced by the same ochratoxinogenic fungi, although this remains to be clarified. The described differences in the observed porcine nephropathy (mainly intranuclear inclusions and myelin-like figures in the proximal tubular epithelial cells, as well as vascular lesions) are probably due to some interference between ochratoxin A and other mycotoxins.
The major lesions are observed in the mitochondria and lipoprotein membranes of proximal tubular cells in the initial stages of this nephropathy, where characteristic macroscopical lesions in kidneys are not clearly defined. A large number of collagen fibrils in the interstitium, and thickened basement tubular membranes, were established at later stages when characteristic macroscopical changes in kidneys were more pronounced. The early changes in the mitochondria, as well lost membrane integrity of the cell organelles, seem to be the mean reason for the death of the proximal tubular cells.
References
BOZIC, Z., V. DUANCIC, M. BELICZA, O. KRAUS, I. SKLJAROV (1995): Balcan endemic nephropathy: still a mysterious disease. Eur. J. Epidemiol. 11, 235-238.
COSYNS, J. P., M. JADOUL, J. P. SQUIFFLET, J. F. DE PLAEN, D. FERLUGA, C. VAN YEPERSELE DE STRIHOU (1994): Chinese herbs nephropathy: a clue to Balkan endemic nephropathy. Kidney Int. 45, 1680-1688.
CREPPY, E. E., F. C. STORMER, D. KERN, R. ROSCHENTHALER, G. DIRHEIMER (1983): Effects of ochratoxin A metabolites on yeast phenylalanyl-tRNA synthetase and on the growth and "in vivo" protein synthesis of hepatoma cells. Chem. biol. Interact. 47, 239-247.
DIMITROV, T. S. (1984): Balkan endemic nephropathy. Publishing house of the Bulgarian Academy of Sciences, Sofia.
ELLING, F., J. P. NELSEN, E. B. LILLEHOJ, M. S. THOMASSEN, F. C. STORMER (1985): Ochratoxin A - induced porcine nephropathy: enzyme and ultrastructural changes after short-term exposure. Toxicon 23, 247-254.
FERLUGA, D., A. HVALA, A. VIZJAK, S. TRNACEVIC, A. HALILBASIC (1991): Renal function, protein excretion, and pathology of Balkan endemic nephropathy. III. Light and electron microscopic studies. Kidney Int. 40 (Suppl 34), 57-67.
FERLUGA, D., A. VIZJAK, A. HVALA, A. VODOVNIC, S. TRNACEVIC, A. HALILBASIC (1996): A kidney biopsy study of early endemic nephropathy. In: Endemic nephropathy in Croatia. (D. Cvoriscec, S. Ceovic, A. Stavljenic-Rukavina, Eds.). Academia Croatica Scientiarum Medicarum. Zagreb. pp. 43-72.
GOLINSKI, P., K. HULT, J. GRABARKIEWICZ-SZCZESNA, J. CHELKOWSKI, P. KNEBLEWSKI, K. SZEBIOTKO (1984): Mycotoxic porcine nephropathy and spontaneous occurence of ochratoxin A residues in kidneys and blood of Polish swine. Appl. Environ. Microbiol. 47, 1210-1212.
HVALA, A., T. KOBENETER, D. FERLUGA, A. VIZJAK, S. TRNACEVIC, A. HALILBASIC (1993): Ultrastructure of tubular basement membrane and basement membrane of peritubular capillaries in Balkan endemic nephropathy. In: Proceeding of Multinational Congress on Electron Microscopy. Parma. pp. 201-202.
HULT, K., E. HOKBY, S. GATENBERG, L. RUTQVIST (1980): Ochratoxin A in blood from slaughter pigs in Sweden: Use in evaluation of toxin content of consumed feed. Appl. Environ. Microbiol. 39, 828-830.
MEISNER, N., S. CHAN (1974): Ochratoxin A, an inhibitor of mitochondrial transport system. Biochem. New York 13, 2795-2800.
MORTENSEN, H. P., B. HALD, A. MADSEN (1983): Feeding experiments with ochratoxin A contaminated barley for bacon pigs. 5. Ochratoxin A in pig blood. Acta Agric. Scand. 33, 235-239.
STOEV, S. D. (1992): Mycotoxic nephropathy in pigs - aetiology, pathogenesis, clinicomorphological and ultrastructural changes. Vet. science 24, 85-90.
STOEV, S. D., D. STOJKOV, T. K. PETKOVA-BOCHAROVA (1994): Mycotoxic nephropathy (ochratoxicosis) in swine. Proceedings of the 8th International Congress on Animal Hygiene. St. Paul. Minnesota, USA. pp. 100-103.
STORMER, F. C. (1992): Ochratoxin A - a mycotoxin of concern. In: Handbook of Applied Mycology. vol. 5, Mycotoxins in Ecological Systems (D. Bhatnagar, E. B. Lillehoj, D. K. Arora, Eds.). Marcel Dekker Inc. pp. 403-431.
Received: 20 February 1997
Accepted: 11 March 1998
STOEV, S. D., N. GROZEVA, B. HALD: Ultrastrukturna i toksikoloska istrazivanja spontanih slucajeva nefropatije svinja u Bugarskoj. Vet. arhiv 68, 39-49, 1998.
SAZETAK
"Povecani, mrljasti ili blijedi bubrezi", nadeni prilikom inspekcije mesa, uzeti su od 50 zaklanih svinja te im je istrazena ultrastruktura u svrhu utvrdivanja naravi nefropatije u Bugarskoj. Tipicne rane ultrastrukturne promjene ustanovljene su u epitelu proksimalnih tubula, a odnosile su se na osebujne lezije u mitohondrijima, koje su se ocitovale u smanjenju mitohondrijskih krista, gubitkom integriteta njihovih membrana, pojavom tvorevina slicnih mijelinu i eletronski gustih tvorevina u mnogim mitohondrijima. Nadalje su ustanovljene specificne intranuklearne uklopine, a takoder i degenerativne promjene u endotelu kapilara. U kasnijem stupnju naden je velik broj kolagenskih fibrila u intersticiju i zadebljanje bazalnih tubularnih membrana. Nefrotoksicni ohratoksin A ustanovljen je u svim uzorcima seruma podrijetlom od istih svinja.
Kljucne rijeci: nefropatija, ohratoksin A, ultrastrukturne promjene, svinja, bubreg, Bugarska